We live our lives inculcating many theories, ideas, and inventions of the past. Our days are planned by internalising on the fact that there is something called day and night. We walk around knowing that all of a sudden, we won’t start to fly because gravity is keeping us grounded. And it’s been proven that we won’t fall off the edge of the earth because it is round. All these internalised facts and many more help us get by our day.
Our lives are shaped because of many inventions and discoveries which seemed astonishing in their times, but today they are important for our understanding of the world that surrounds us. The discovery of the atom and the invention of the computing machine are examples of such scientific happenings. Imagine a world without gadgets and electricity.
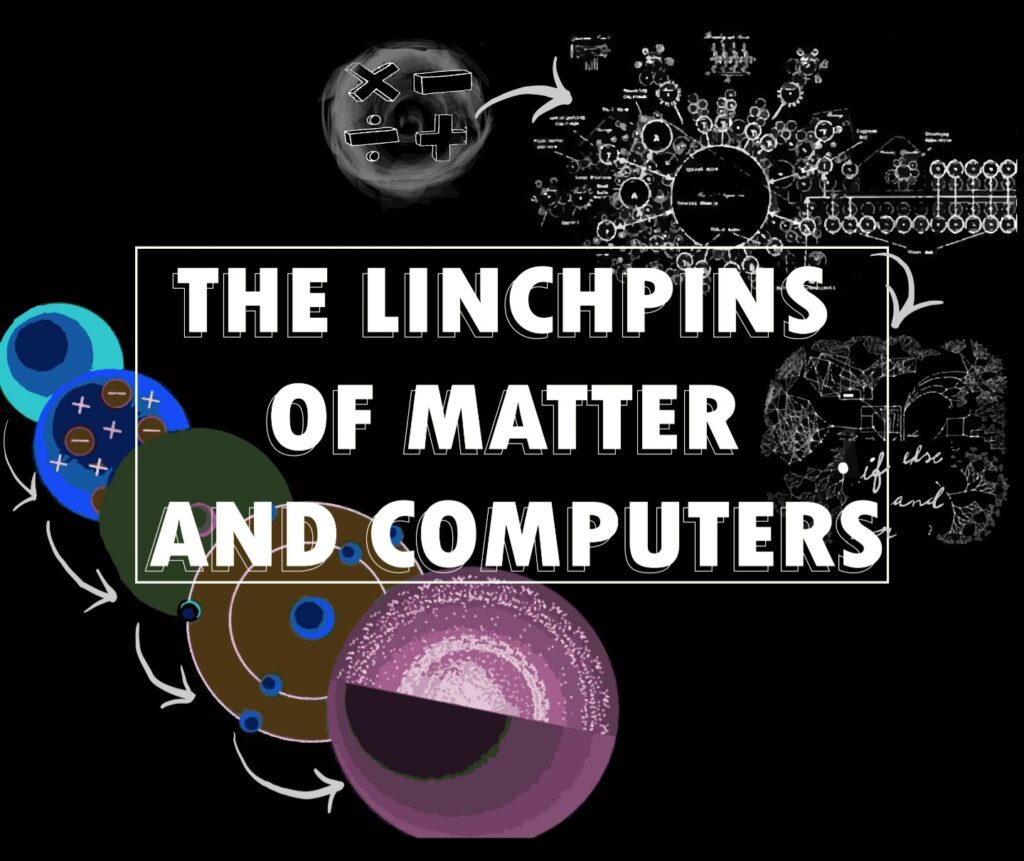
DIVIDING THE INDIVISIBLE
Atomic theories have long been an integral part of science, with many scientists producing their approaches, often through a combination of experimentation, as well as pre-existing theories. The way atoms are understood today is a result of multiple developments made on the subject for centuries, especially between the 18th and 20th centuries.
The atomic theory has had an evolution of sorts, wherein each new theory incorporates aspects of its predecessor, while also containing new additions and possible changes or corrections in the previous one.
The conception of atoms as the foundation of all matter occurred as early as the 5th century in Greece, and later in India, in a school of thought now known as Atomism. Unlike the current discourse surrounding Atomism, as conceived by the Greeks and Indians, was instead done more philosophically in an attempt to know more about the universe and what it consists of. It is this theory that was then adopted and studied by the scientific community later on.
John Dalton
The first Atomic theory that changed and influenced its successors was the one by John Dalton in 1803. While Dalton’s theory bears little to no resemblance to what is accepted today, it made a few noteworthy discoveries that differed from the theory stated by the Greeks.
Dalton has disproved the Atomist notion of all atoms being alike, as he believed that like atoms repel each other, while unlike atoms attract each other. While this theory was later proven to be false, it introduced the idea of atoms differing from each other in ways such as size and mass. Additionally, he also stated that to find the relative mass of an atom, one must find out the number of atoms that exist in each element. Therefore, Dalton laid the foundation of the future of the Atomic theory, which in itself was influenced by the works of thinkers centuries prior.
Plum Pudding Model
The theory that came after Dalton’s was the one by J.J. Thomson, who too, introduced new developments regarding the structure and composition of atoms in 1904. Here, the latter is of more importance, as this deviates from the notion of the atom being the foundation of all matter.
Thomson believed that atoms were divisible and consisted of positive and negative charges which could interact with each other electromagnetically. Furthermore, the theory states that atoms consist of a “sea of uniform positive charge” with electrons distributed within it. He also suggested that atoms were not inert, as opposed to previously held notions. This hence introduced the study of its motion as well.
Nuclear Model
Having discovered positive and negative charges within the atom, Ernest Rutherford, in 1911, expanded on this by describing the exact structure of the atom, with the inclusion of a new component: the nucleus.
His theory was devised with his famous Gold Foil experiment, wherein he shot an alpha ray at a strip of gold foil and found that some of it deflected while some went through the foil. This led him to two conclusions. First, the atom consisted of some empty space which allowed parts of the ray to pass through the foil, as they were not blocked by anything. Second, the rays that deflected did so because they came in contact with something of a like charge (positive, in this case, due to the positive charge of the alpha ray) causing them to repel said component.
He then decided on a new structure of the atom, wherein a positively charged nucleus lies at the centre, and around it were negatively charged components. This closely resembles Thomson’s theory, as it too mentions positive and negative charges. Rutherford’s model of the atom, however, introduces the idea of the positive charge existing in the middle of the atom, with the negative charges surrounding it, as opposed to the atom consisting of a sea of positive charges.
Planetary Model
In 1915, Niels Bohr had added to Rutherford’s model of the atom, leading to this new model often being labelled as the Bohr-Rutherford model due to how similar they are. More than a correction of Rutherford’s model, it was a modification, as the general structure of the atom stays consistent. Bohr added to the Nuclear Model by stating that the negatively charged electrons orbit around the nucleus, much like planets orbiting around the sun. This is what led to it being named the Planetary Model.
Similarly, Bohr also discovered a pull between the positively charged nucleus and the electrons around it, which he again described in terms of the solar system, by comparing it to the gravitational force that exists in the solar system.
Bohr also theorised about the size of atoms, by claiming that these depend on the energy of the orbits: the smaller the orbit, the lesser the energy. He also believed that each orbit could only hold a certain number of electrons. Once an orbit was full, electrons would occupy another orbit outside the previous one.
There is an observable pattern among these theories, as they draw on the ideas from the theories prior. What started as a possible answer to a philosophical question laid the groundwork for a plethora of theories.
Based on the initial atomic model of the ‘smallest particle,’ it led to other theories being formed. Each of the refined theories led us closer to the now definitive model of the atom.
Shifting from Calculating to Computing
Did you listen to music today? Or play some games on your devices? Were you scrolling through some app before jumping on this article? Well, if you have done any of the above, take some time off your screens and thank Ada Augusta Lovelace. Hailed by many as the world’s first programmer, Lovelace was a visionary way ahead of her times. She proposed the shift from calculating to computing while working with Charles Babbage on Analytical Engine. She approached her surroundings and mathematical interests through what she called ‘poetic science’.
The Analytical Engine was the successor of the Difference engine, which was the first automatic mechanical calculator. While working on the Difference Engine, Babbage wanted to move beyond tabulating polynomial functions. Analytical Engine was a general-purpose mechanical computer which was programmable and had the memory. Unfortunately, Babbage never constructed any of his machines due to lack of funds and disputes with his engineer. The world would still have to wait for a century to use the first computing devices. Nevertheless, the symptoms of the ripples created by Analytical Engine remain central to the early understanding of codes and computing machines.
In 1843, Ada Lovelace worked on translating Luigi Menabrea’s paper on Analytical Engine from French to English. It was in this translation that Lovelace proposed the extended uses of Analytical Engine. She added notes explaining the working of the Engine, making the paper more detailed than the French version. Lovelace also describes an algorithm to calculate the Bernoulli numbers— a sequence of numbers used in mathematical calculations. This algorithm worked on codes and is the reason why Lovelace is credited to be the world’s first programmer. Though there are disputes among the historians as to the degree of influence Babbage had in the compilation of these codes, there are other aspects concerning the Engine where Lovelace’s originality of thought remains undisputed.
Along with the algorithms in her notes, Lovelace also mentions how codes can be compiled for the machine to operate letters, symbols, and numbers. This is the preliminary foundation of all the gadgets we use today. In those notes, Ada put the ability of the Analytical Engine to solve problems of any complexity in the centre. Owing to her quest of making mathematics a central part of everyday life, she believed that if harmony and pitch of musical composition could be adapted in mathematical expressions, then the Engine would be able to compose ‘scientific pieces of music’. In contemporary times, almost two centuries later Lovelace’s predictions, music producers around the world are using artificial intelligence to produce musical harmonies. Since Babbage could never complete the construction of the Analytical Engine; Ada Lovelace’s algorithms and proposals could never be tested. However, present researchers regard Babbage’s Engine as an early model of computer and Lovelace’s algorithms and codes as predecessors of computer software.
For Babbage, engines were bound with numbers, numbers representing a quantity. Lovelace however, believed that numbers could be used to describe other entities apart from quantifiable numbers. She proposed that various symbols and entities could be represented through codes and algorithms. So an engine which manipulates numbers can manipulate things like letters, sounds (musical notes) and colours according to the rules (algorithm) we feed it. This shift in the idea of a machine as one working with numbers or ‘a number cruncher’ to one operating a symbol is credited to Lovelace. This also signifies the fundamental shift from calculation to computation. This underplay of algorithms and codes connected with symbols is the basic functioning of digital displays in our gadgets.
Hence, Lovelace became the first person to recognise that the machine had applications apart from the pure calculations. She envisioned the reach of computers to go beyond mathematics. Her concerns of everyday life and an inclination to the late 18th and early 19th-century romanticism urged her in including computing in everyday life as well as in scientific endeavours of the Industrial Revolution. The inspiration for Lovelace’s proposal came from the complicated looms of the textile industry. She saw the machines weaving patterns of flowers and other designs in various textiles. Later, she extended the same to Analytical Engine; the punch card system of Babbage’s design was similar to machines used in the textile industry. Lovelace observed the algebraic pattern made by the Engine while carrying out calculations and proposed using these patterns for further computations.
Charles Babbage worked and invented the earliest version of the computer in a very charged social environment. At the time of its invention, the Analytical Engine wasn’t received with much popularity, and many of Babbage’s contemporaries dismissed it. The role of Ada Lovelace then, as an advocate and supporter of the invention becomes more crucial. She didn’t only support the project, but also intellectually engaged with it, and took it a step further than Babbage’s proposal. Her imagination did what Babbage’s rationality couldn’t: putting mathematics and logic of symbols by the side of science. Today, almost all machines rely on coded symbols and algorithms to function. Although Lovelace couldn’t herself see her idea taking shape due to the limitations of her time, two centuries later, her ideas made it possible for me to write this article and for you to read it. So next year on the second Sunday of October, take a moment to appreciate your gadgets and the genius imagination of Lovelace behind this technology because that day is celebrated as Ada Lovelace day!
Just as all things change and evolve through time, so do inventions and theories in science. Being a field that seeks to create, innovate and ponder, science, by nature, is in a constant state of flux. Brilliant minds all over the world seek to make developments in the field that will not only answer the most vital questions such as what all things are made of but also create new inventions to aid us as times keep changing.
Science, therefore, becomes a system of its own that follows its path of evolution, wherein man himself decides the next course of action that leads to new developments. Science is never static, and can never be so. The questions it seeks to answer and the problems it aims to solve are limitless, which means we will always know and have more than we did before. In other words, it is a journey with no specific destination, but rather multiple checkpoints, all of a great deal of importance in their particular time and place.
Written by Lavya Joshi and Kalyani for MTTN
Edited by Kaavya Azad for MTTN
Featured Image by Vanshika Chanani for MTTN
Leave a Reply
You must be logged in to post a comment.